Abstract

Background: Patients with TP53mut AML or with del(17p) experience refractoriness to conventional intensive chemotherapy and dismal outcomes. Treatment with 10-day decitabine may be beneficial in these patients. The utility of augmenting a hypomethylating agent (HMA) backbone with the BCL-2 inhibitor, venetoclax (VEN), in these patients is under debate, as recent studies suggest TP53mut may also confer resistance to VEN. The aim of this study was to report response, survival, and toxicity of HMA with and without addition of VEN in matched cohorts of del(17p) and TP53mut AML.
Patients & Methods: We analyzed 47 patients with TP53mut or del(17p) AML treated with HMA +/- VEN from January 2016 to December 2021 at VCU Massey Cancer Center. Baseline patient and disease characteristics including molecular and cytogenetic profiling, treatment regimens, responses, and survival data were obtained via retrospective chart review. Patients were considered evaluable for response if there was a bone marrow biopsy following a minimum of 28 days of treatment. The event for calculating the overall survival (OS) was the date of death. Patients were otherwise censored at the date of last contact.
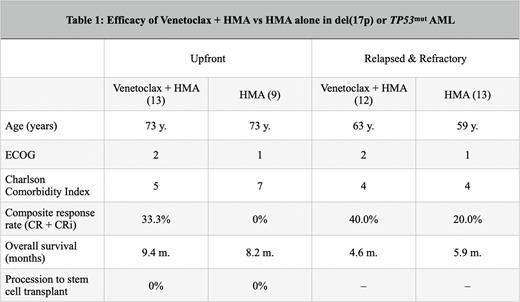
Results: Thirteen patients with TP53mut/del(17p) AML were treated with HMA + VEN in the first-line setting. The median age at diagnosis was 73 years, median ECOG score 2, and the median Charlson Comorbidity Index (CCI) score was 5. The composite response rate (CRR; CR + CRi) was 33.3% in those evaluable (1 CR, 3 CRi). The median OS was 9.4 months and no patients proceeded to allogeneic stem cell transplant (alloSCT). In the HMA monotherapy cohort, nine patients were treated with decitabine (33.3%) or azacitidine (66.6%) with TP53mut or del(17p). The median age was 73 years, median ECOG score 1, and median CCI score 7. No patients achieved a CR or CRi. The median OS was 8.2 months and no patients proceeded to alloSCT. There was no significant difference in survival with or without the addition of VEN in the first-line setting (p = 0.8735).
There were 12 patients in the relapsed/refractory (R/R) cohort treated with HMA/VEN that did not previously receive this therapy upfront. The median age was 63 years, median ECOG score was 2, and the median CCI score was 4. The CRR was 40.0% in those evaluable (2 CRi, 2 CRi). The median OS was 4.6 months. In the relapsed/refractory HMA monotherapy cohort, 13 patients were identified. The median age was 59 years, the median ECOG score was 1, and the median CCI score was 4. The CRR was 20.0% (1 CR, 1 CRi) in those evaluable. The median OS was 5.9 months.
Conclusions: While HMA + VEN in the first-line setting did translate to a higher CRR in TP53mut disease compared to HMA monotherapy, our data suggest no clear additional survival benefit with the addition of VEN. These patients continue to experience refractory disease, inability to proceed with alloHSCT, and poor survival even with augmentation of the HMA backbone by BCL-2 inhibition. These findings suggest cautious selection for VEN candidates and highlight the need for novel therapeutic interventions for TP53mut disease. Larger studies with propensity score-based techniques are needed to confirm these findings.
Disclosures
Maher:BMS: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal